|
|
| Compound | Antrodin C |
| Animal species | rat intestinal bacteria |
| Metabolism parameters |
Oral administration Intravenous administration |
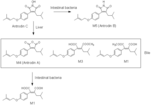
| Metabolites |
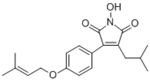
Antrodin C
Antrodin B Antrodin A (Z)-3-(methoxycarbonyl)-5-methyl-2-(4-((3-methylbut-2-en-1-yl)oxy)phenyl)hex-2-enoic acid (Z)-2-(2-methoxy-1-(4-((3-methylbut-2-en-1-yl)oxy)phenyl)-2-oxoethylidene)-4-methylpentanoic acid 2-isobutyl-3-(4-((3-methylbut-2-en-1-yl)oxy)phenyl)maleic acid |
| Crude drug | |
| References | 1) Zuo F., Wada A., Ma C. M., Nakamura N., and Hattori M., Metabolism and disposition of antrodins C, a cytotoxic major principle from the mycelium of Antrodia cinnamomea, in rats. Unpublished results. |
| Remarks | ・LC/MS-MS Condition. The LC/MS-MS equipment comprised a column containing TSK gel ODS-80 Ts (particle size, 5 µm; 4.6×150 mm i.d., Tosoh Co., Tokyo, Japan). Samples were eluted through a column with 0.1% AcOH and acetonitrile (35:65) at a flow rate of 1 ml/min at 30ºC. The standard negative ion mode was selected under the following conditions: full scan range, 50-800 m/z; scan resolution, 13000 m/z /sec; nebulizer, 50.0 psi; dry gas, 10.0 l/min; dry temperature, 360ºC. ・Animal Experiments. Male Wistar rats (9 weeks old) purchased from SLC Co. (Hamamastu, Japan), were fed with standard laboratory chow for one week, fasted overnight and given free access to water before drug administration. Urine and feces samples were collected while the rats remained isolated in metabolic cages. The animals were light anesthetized with diethyl ether during surgical procedures. Bile samples (n=5) was collected by cannulating a polyethylene tube (PE-10) into a rat bile duct at intervals of 0, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36 and 48 h after oral (50 mg/kg) and intravenous (10 mg/kg) administration of antrodin C. The blood sample was collected from the inferior vena cava using a heparinized injector when the abdomen was exposed by a midline abdominal incision after administration. The blood samples were centrifuged at 8000×g for 15 min to separate the plasma, and then all samples were stored at –20ºC for later analysis. ・Pharmacokinetic Analysis. The concentration-time data in rat bile (n=5) were computer fitted using a program of Pharmacokineitics 3p97 edited by the Mathematics Pharmacological Committee, Chinese Pharmacological Society. The following pharmacokinetic parameters were obtained: half-time of absorption phase (t1/2 (Kα)) and half-time of elimination phase (t1/2 (Kβ)) in the bile samples after oral administration of antrodin C at a dose of 50 mg/kg. The area under the concentration–time curve (AUC(i.v.) and AUC(p.o.)) was calculated by the statistical moment method of non-compartmental pharmacokinetic analysis. Clearance (Clm, b) and absolute bioavailability (Fm, b) were calculated by an equations as follows: Clm, b (ml/h・kg) = Dose (i.v.)/ AUC (i.v.) and Fm, b (%) = AUC (p.o.) ・ Dose (i.v.)/ [AUC (i.v.) ・ Dose (p.o.)]. Data were expressed as the mean and standard deviation (S.D.) for each group. |

