|
|
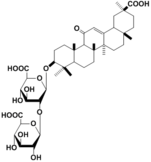
| 化合物名 | Glycyrrhizin |
| 動物種 | 腸内細菌代謝 ヒト腸内細菌フローラ、ヒト腸内細菌分離株 Eubacterium, Clostridium innocum |
| 代謝パラメータ | |
| 代謝物 |
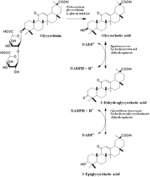
Glycyrrhizin
Glycyrrhetic acid 3-Dehydroglycyrrhetic acid 3-Epiglycyrrhetic acid Glycyrrhizin Glycyrrhetic acid 3-O-glucuronide |
| 関連生薬 | 甘草 |
| 参考文献 | 1) Hattori M., Sakamoto T., Kobashi K. and Namba T.: Metabolism of glycyrrhizin byhuman intestinal flora. Planta Med., 48, 38-42 (1983). 2) Hattori M., Sakamoto T., Yamagishi T., Sakamoto K., Konishi and Namba T.: Metabolism of glycyrrhizin by human intestinal flora. II. Isolation and characterization of human intestinal bacteria capable of metabolizing glycyrrhizin and related compounds. Chem. Pharm. Bull., 33, 210-217 (1985). 3) Akao T., Akao T., Hattori M., Namba T. and Kobashi K.: 3β-Hydroxycholanate dehydrogenase of Ruminococcus sp. from human intestinal bacteria. J. Biochem. 99, 1425-1431 (1986). 4) Akao T., Akao T., Hattori M., Namba T. and Kobashi K.: Enzymes involved in the formation of 3β,7β-dihydroxy-12-oxo-5β-cholanic acid from dehydrocholic acid by Ruminococcus sp. obtained from human intestine. Biochim. Biophys. Acta., 921, 275-280 (1987). 5) Akao T., Akao T., Hattori M., Namba T. and Kobashi K.: Purification and properties of 3α-hydroxyglycyrrhetinate dehydrogenase of Clostridium innocuum from human intestine. J. Biochem., 103, 504-507 (1988). 6) Akao T., Aoyama M., Akao T., Hattori M., Imai Y., Namba T., Tezuka Y., Kikuchi T. and Kobashi K.: Metabolism of glycyrrhetic acid by rat liver microsomes. II. 22α- and 24-hydroxylation. Biochem. Pharmacol., 40, 291-296 (1990). 7) Akao T., Akao T., Hattori M., Kanaoka M., Yamamoto K., Namba T. and Kobashi K.: Hydrolysis of glycyrrhizin to glycyrrhetyl monoglucuronide by lysosomal β-D-glucuronidase of animal livers. Biochem. Pharmacol., 41, 1025-1029 (1991). 8) Akao T., Akao T., Hattori M., Namba T. and Kobashi K.: Inhibitory effects of glycyrrhetic acid and the related compounds on 3a-hydroxysteroid dehydrogenase of rat liver cytosol. Chem. Pharm. Bull., 40, 1208-1210 (1992). |
| 論文備考 | ※Incubation of glycyrrhizin with an intestinal bacterial mixture An intestinal bacterial mixture (40 ml) and GAM broth (310 ml) were mixed thoroughly and incubated at 37° C for 24 hr in an anaerobic jar, in which air had been replaced with CO2 gas in the presence of activated steel wool. Glycyrrhizin (mono ammonium, 400 mg) was anaerobically incubated at 37°C for 24 hr in a similar fashion. The mixture were adjusted to pH ca. 1 with HC1, and extracted six times with CHCl3 (200 ml each). The CHCl3 phase was washed with water, then concentrated to ca. 20 ml in vacuo. The mixture was applied to a column of silica gel (2.4 x 44 cm). The column was washed with CHCl3, and eluted with CHCl3-MeOH (100:1). Fractions of 3.6 ml/tube were collected. Fr. 102-112 (metabolite I) and fr. 125-136 (metabolite II) were separately pooled and evaporated to dryness in vacuo. The metabolites I (ca. 150 mg) and II (ca. 43 mg) were then purified by repeated crystallization from CHCl3-petroleum ether. [Hattori et al., Planta Med., 48, 38-42 (1983)] ※Animals and livers. Wistar strains of male and female rats at 4-14 weeks of age and ddy strains of male mice at 4-6 weeks of age were used. One bovine and three porcine livers were purchased from Nippon Ham Co. (Osaka, Japan). Human liver tissues were obtained from non-involved part of the resected specimen of the metastatic liver tumors. [Akao et al., Biochem. Pharmacol., 40, 291-296 (1990)] ※Preparation of subcellular fractions and lysosomes Fresh livers obtained from rat, mouse and human were used for preparation without storage, and livers from cattle and porcine after storage at -20° for a few months. Liver homogenates in 0.25 M sucrose were separated into nuclear, mitochondrial, lysosomal, microsomal and soluble fractions. Hepatic lysosomes were prepared by centrifuging liver homogenates in 0.15 MKC1 at 5000 g for 10 min and then the supernatant at 10,000g for 20 min. Lysosomes suspended in 50 mM potassium phosphate buffer (pH 7.0) were sonicated and then centrifuged at 100,000 g for 90 min to obtain a clear supernatant. [Akao et al., Biochem. Pharmacol., 40, 291-296 (1990)] ※Isolation of glycyrrhetic acid 3-O-glucuronide produced from glycyrrhizin by rat liver lysosomes. The supernatant (230 mg of protein) of the sonicated lysosomes from four male rats (14 weeks old) was incubated with 26 μmol of glycyrrhizin in 100 mL of 0.1 M acetate buffer (pH 5.6). After incubation at 37° for 1 hr, the reaction was stopped by the addition of 1 M HC1. It was extracted twice with an equal volume of ethyl acetate. After evaporating the ethyl acetate phase, glycyrrhetic acid 3-O-glucuronide was isolated as powder by preparative TLC, though glycyrrhizin was also detected on the plate. [Akao et al., Biochem. Pharmacol., 40, 291-296 (1990)] |

