|
|
| 化合物名 | Arbiflorin |
| 動物種 | ヒト腸内細菌フローラ |
| 代謝パラメータ | |
| 代謝物 |
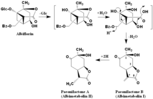
Albiflorin
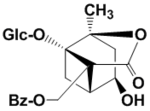
((1R,3R,4R,6S,9S)-1,4-dihydroxy-6-methyl-8-oxo-7-oxatricyclo[4.3.0.03,9]nonan-9-yl)methyl benzoate (1R,2S,4R,5R,6S)-6-((benzoyloxy)methyl)-1,2,4-trihydroxy-2-methylbicyclo[3.1.1]heptane-6-carboxylic acid Paeonilactone B(Albimetabolin I) Paeonilactone A(Albimetabolin II) |
| 関連生薬 | 芍薬 |
| 参考文献 | 1) Hattori M., Shu Y. Z., Kobashi K. and Namba T.: Metabolism of albiflorin by human intestinal bacteria. J. Med. Pharm. Soc. Wakan-Yaku, 2, 398-404 (1985). and analyzed by TLC-densitometry. |
| 論文備考 | PIncubation of albiflorin with a fecal suspension Albiflorin (85 mg) was added to a fecal suspension (100 ml). The mixture was incubated for 30 hrs at 37°C in an anaerobic jar, in which the air had been replaced with oxygen-free carbon dioxide in the presence of activated steel wool (steel wool method), and then extracted four times with ethylacetate (AcOEt, 100 ml each). The solution was evaporated in vacuo to give an oily residue (ca. 600 mg). The residue was applied to a column of silica gel (40 g, 1.9 cm i.d. x 24 cm). The column was washed with benzene (500 ml) and benzene-CHCl3 (1 : 1, 500 ml), and then eluted with CHC13. The fractions (50 ml each) containing metabolites were pooled and evaporated to dryness in vacuo. The crude metabolites were further purified by repeated crystallization and preparative TLC. Al and A2 were obtained in yields of 11 mg (29 %) and 1 mg (2 %), respectively. [Hattori et al., J. Med. Pharm. Soc. Wakan-Yaku, 2, 398-404 (1985)] |

