|

|
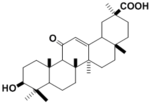
| 化合物名 | Glycyrrhetic acid |
| 動物種 | 腸内細菌代謝 ヒト腸内細菌フローラ、ヒト腸内細菌株 Clostridium innocuum ES24-06, Ruminococcus sp. POl-3 |
| 代謝パラメータ | |
| 代謝物 |
Glycyrrhetic acid
3-Dehydroglycyrrhetic acid 3-Epiglycyrrhetic acid |
| 関連生薬 | 甘草 |
| 参考文献 | 1) Hattori M., Sakamoto T., Kobashi K. and Namba T.: Metabolism of glycyrrhizin by human intestinal flora. Planta Med., 48, 38-42 (1983). 2) Hattori M., Sakamoto T., Yamagishi T., Sakamoto K., Konishi and Namba T.: Metabolism of glycyrrhizin by human intestinal flora. II. Isolation and characterization of human intestinal bacteria capable of metabolizing glycyrrhizin and related compounds. Chem. Pharm. Bull., 33, 210-217 (1985). |
| 論文備考 | ※Preparation of a human intestinal bacterial mixture Fresh faeces were immediately transferred into a vinyl bag filled with CO2 gas. The bag was then pressed by hand to uniformly mix the contents. The faeces thus obtained was suspended in five volumes of GAM broth or PGPY broth, then centrifuged at 16 x g for 1 min to eliminate the residue. The upper phase was used in all experiments as an intestinal bacterial mixture. [Hattori et al., Planta Med., 48, 38-42 (1983)] ※Incubation of 18β-glycyrrhetic acid with an intestinal bacterial mixture 18β-Glycyrrhetic acid (272 mg) dissolved in EtOH (14 ml) and an intestinal bacterial mixture (200 ml) were added to GAM broth (4000 ml), which were then anaerobically incubated at 37° C for 48 hr. The culture medium was adjusted to pH 1 and extracted 4 times with AcOEt (2000 ml each). The AcOEt phase was washed with 2 % NaCl and concentrated to a small volume in vacuo. The metabolic mixture obtained was applied to a silica gel column (2.4 x 44 cm). The column was first eluted with CHCl3 (5 1) and fractions of 500 ml/flask were collected. The fourth fraction was evaporated to dryness in vacuo and the residue was further purified by repeated preparative TLC and crystallization from CHCl3-petroleum ether. Yield 2 mg. mp > 300°C, UV λmax (EtOH): 250 nm, MS: m/z 468 (M+, 29%), 453 (15%), 440 (15%), 422 (17%), 303 (73%), 262 (75 %), 216 (12 %), 135 (100 %), IR λmax (KBr): 3310 (COOH), 1726 (C=O) cm-1. 1NMR (DMSO-d6) δ: 0.79, 0.98, 1.03, 1.09, 1.12, 1.16, 1.38 (each 3H, each s, C-CH3), 5.48 (1H, s, C = CH). This compound was identified to be 3-dehydro-18β-glycyrrhetic acid. The column was next eluted with CHCl3-MeOH (100:1). Fractions of 10 ml/tube were collected, monitoring by silica gel TLC. Fr. 1-10 were pooled and evaporated to dryness in vacuo. The precipitate (ca. 25 mg) was purified by preparative TLC and by repeated crystallization from CHCl3-benzene-MeOH to give pure 3-epi-18β-glycyrrhetic acid (3.3 mg). Fr. 11-39 contained two components, which were then separated to give 3-epi-18β-glycyrrhetic acid (ca. 1 mg) and 18β-glycyrrhetic acid (31 mg) by rechromatography. Fr. 40-49) were work up in a similar fashion. The precipitate (ca. 144 mg) was crystallized from CHCl3-petroleum ether to recover 18β-glycyrrhetic acid (23 mg). [Hattori et al., Planta Med., 48, 38-42 (1983)] ※Isolation of 3-epi-18β-glycyrrhetic acid Precultured Clostridium innocuum ES24-06 was inoculated into GAM broth (200 ml) and cultured for 5 h at 37 °C. 18α-Glycyrrhetic acid (94 mg) in EtOH (20 ml) was added to the culture medium, which was further incubated for 10 h at 37 °C. Then 1 N HC1 (1000 ml) and NaCl (150 g) were added, and the medium was extracted three times with EtOAc (1.5 1). The EtOAc solution was concentrated to a volume of ca. 1000 ml, washed with a saturated NaCl solution (500 ml), dried over Na2SO4, and then evaporated to dryness in vacuo below 40 °C. The residue was dissolved in a small volume of CHCl3 and the solution was applied to a column of silica gel (36 x 2 cm). The column was washed with CHCl3 (700 ml) and eluted with CHCl3-MeOH (100:1). Fractions I-IV (100 ml each) were pooled, evaporated to dryness in vacuo, and washed with H2O-EtOH. The precipitate (57.4 mg) was purified by preparative thin layer chromatography (Merck, Kieselgel 60 F254 S, 2 mm layer thickness) using solvent system F and the product was crystallized from n-PrOH-petroleum ether to give colorless prisms (5.1 mg). mp >300°C, Anal. Calcd for C30H46O4: C, 76.55; H, 9.85. Found: C, 76.54; H, 9.63. 1H-NMR (DMSO-d6) δ: 0.65, 0.77, 0.84, 1.04, 1.13, 1.16, 1.35 (each 3H, each s, C-CH3), 2.8 (1H, brs, CH-OH), 5.33 (1H, s, C = CH). UV λmax: 244 nm. MS m/z: 470 (M+, 2%), 452 (4%), 437 (5%), 303 (100%), 262 (18%), 257 (15%), 175 (15%), 135 (60%). IR γmax (KBr): 3480 (OH), 1709 (COOH), 1650 (conjugated C = O), 1615 (conjugated C = C) cm-1. [Hattori et al., Chem. Pharm. Bull., 33, 210-217 (1985)] ※Reduction of 3-dehydroglycyrrhetic acid A human intestinal bacterium or a bacterial mixture was anaerobically cultured for 48 h at 37 °C in GAM broth (20 ml) containing 3-dehydroglycyrrhetic acid (1 mmol). A-portion (10 ml) of the culture was acidified to pH 1 with HC1 and extracted with ethyl acetate (EtOAc, 10 ml x 2) after adding NaCl (2g). The EtOAc solution was concentrated to a volume of 1 ml and an aliquot (12 μl) of it was spotted on a TLC plate, which was then developed with solvent system F. Metabolites glycyrrhetic acid and 3-epi-glycyrrhetic acid were quantitatively analyzed by TLC-densitometry. [Hattori et al., Chem. Pharm. Bull., 33, 210-217 (1985)] |

