|

|
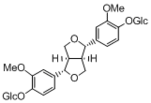
Crude drugs
Kampo formulas
Experimental materials in Wakanyaku Library
Information on genetic analysis
Information on chemical analysis
Information of bioassay
Metabolism information on chemical compounds included in crude drugs
Exploratory Research
Links
Traditional Medical & Pharmaceutical Database
Section of Pharmacognosy,
Division of Medicinal Resources,
Department of Research and Development,
Institute of Natural Medicine,
University of Toyama
2630 Sugitani, Toyama 930-0194 Japan
TEL: +81-76-434-7601
FAX: +81-76-434-5064

