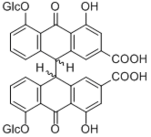
|

|
| 化合物名 | Sennoside A |
| 動物種 | 腸内細菌代謝 ヒト腸内細菌株 Clostridium sphenoides, Eubacterium rectale動物代謝 ラット |
| 代謝パラメータ | |
| 代謝物 |
Sennoside A
Sennidin 8-glucoside Sennidin 8-Glucosylrhein anthrone Rhein anthrone |
| 関連生薬 | センナ , 大黄 |
| 参考文献 | 1) Kobashi K., Nishimura T., Kusaka M., Hattori H. and Namba T.: Metabolism of sennosides by human intestinal bacteria. Planta Med., 40, 225-236 (1980). 2) Hattori M., Kim G., Motoike S., Kobashi K. and Namba T.: Metabolism of sennosides by intestinal flora. Chem. Pharm. Bull., 30, 1338-1346 (1982). 3) Akao T., Akao T., Mibu K., Hattori M., Namba T. and Kobashi K.: Enzymatic reduction of sennidin and sennoside in Peptostreptococcus intermedius. J. Pharmaco-bio Dyn., 8, 800-807 (1985). 4) Akao T., Mibu K., Erabi T., Hattori M., Namba T. and Kobashi K.: Non-enzymatic reduction of sennidins and sennosides by reduced flavin. Chem. Pharm. Bull., 35, 1998-2003 (1987). 5) Hattori M., Namba T., Akao T. and Kobashi K.: Metabolism of sennosides by human intestinal bacteria. Pharmacology, 36 (s-1), 172 -179 (1988). 6) Akao T., Che Q. M., Kobashi K., Yang L., Hattori M. and Namba T.: Isolation of a human intestinal anaerobe, Bifidobacterium sp. strain SEN, capable of hydrolyzing sennosides to sennidins. Applied and Environmental Microbiology, 60, 1041-1043 (1994). 7) Yang L., Akao T., Kobashi K. and Hattori M.: A sennoside-hydrolyzing b-glucosidase from Bifidobacterium sp. strain SEN is inducible. Biol. Pharm. Bull., 19, 701-704 (1996). 8) Yang L., Akao T., Kobashi K. and Hattori M.: Purification and characterization of a novel sennoside-hydrolyzing b-glucosidase from Bifidobacterium sp. strain SEN, a human intestinal anaerobe. Biol. Pharm. Bull., 19, 705-709 (1996). Bull., 19, 705-709 (1996). |
| 論文備考 | ※Preparation of a suspension of rat feces and its supernatant fluid Fresh feces (20 g) of Wistar rats (female, 180-220 g body weight) were suspended in 100 mM phosphate buffer (200 ml, pH 7.3) containing 0.05% cysteine, which had previously been bubbled through with carbon dioxide to eliminate air. The supernatant fluid was prepared by centrifuging the suspension at 10000 rpm for 10 min. [Hattori et al., Chem. Pharm. Bull., 30, 1338-1346 (1982)] ※Incubation of sennoside A with rat feces and quantitative analysis of its metabolites To 5 ml of a suspension of rat feces was added 500 μl of a sennoside A solution (1 mg/ml, dissolved in 100 mM phosphate buffer, pH 7.3). After replacing air in the test tube with carbon dioxide, the mixture was incubated at 37°C for the indicated periods of time. The tube was then immediately cooled and centrifuged at 10000 rpm for 10 min. Next, 2% ethylenediaminetetraacetic acid (EDTA) (0.5 ml), 0.5 n HC1 (0.5 ml) and n-BuOH (2 ml) were added to 2 ml of the upper layer. After vigorous shaking, the mixture was centrifuged at 3000 rpm for 10 min to separate it into two layers. Five μl of the upper layer was applied on a silica gel thin-layer plate (Merck Silica gel 60 F254, layer thickness 0.25 mm). The plate was then developed with a solvent system A, n-PrOH-AcOEt-H2O (4: 4: 3, v/v) containing a few drops of AcOH. The spots on chromatogram were detected under ultraviolet (UV) light and analyzed quantitatively by using a Shimadzu CS-910 chromatoscanner (Shimadzu Seisakusho Ltd., Kyoto). [Hattori et al., Chem. Pharm. Bull., 30, 1338-1346 (1982)] ※Isolation of sennidin A 8-glucoside Sennoside A (1 g) was incubated with a suspension (2.5 1) of rat feces at 37°C for 30-60 min, followed by centrifugation at 10000 rpm for 20 min. The supernatant fluid was acidified with hydrochloric acid to pH 3, and extracted with BuOH (2.5 1). The extract was washed with water, neutralized with triethylamine, and concentrated in vacuo below 37°C to a small volume (1-2 ml), which was then applied to a column (3 x 41 cm) of Sephadex LH 20 (Pharmacia Fine Chemicals) and eluted with 70% methanol. Fractions of 2 ml/tube were collected, and the absorbance at 370 nm was monitored. Fractions were pooled and evaporated to dryness in vacuo. Peak I contained substances mostly derived from feces. Peak II and Peak IV were identified as sennoside A and sennidin A, respectively, by comparing Rf values on TLC and absorption spectra with those of authentic samples. Peak III was further purified by repeated Sephadex LH 20 column chromatography to give 2 mg of a chromatographically homogeneous compound (sennidin A 8-glucoside): Rf =0.43 on TLC developed with solvent system A, UV-VS: λmax, pH 1: 218, 270, 300 (shoulder), 387 nm; pH 7: 220, 270, 365 nm; pH 13: 225, 297 (shoulder), 422 nm. [Hattori et al., Chem. Pharm. Bull., 30, 1338-1346 (1982)] |

