|
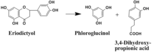
|
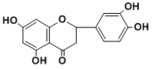
| Compound | Eriodictyol |
| Animal species | human intestinal microflora |
| Metabolism parameters |
Intravenous administration Intravenous administration |
| Metabolites |
Eriodictyol
Phloroglucinol 3,4-Dihydroxy-propionic acid |
| Crude drug | Swertia Herb |
| References | 1) Hattori M., Shu Y. Z., El-Sedawy A. I., Namba T., Kobashi K. and Tomimori T.: Metabolism of homoorientin by intestinal bacteria. J. Nat. Prod., 51, 874-878 (1988). 2) Ange´ Lique Stalmach, William Mullen, Monia Pecorari, Mauro Serafini, and Alan Crozier, Bioavailability of C-linked dihydrochalcone and flavanone glucosides in humans following ingestion of unfermented and fermented rooibos teas. J. Agric. Food Chem., 57, 7104-7111 (2009). DOI:10.1021/jf9011642 3) Jaime A. Yanez, Connie M. Remsberg, Nicole D. Miranda, Karina R. Vega-Villa, Preston K. Andrews and Neal M. Davies, Pharmacokinetics of selected chiral flavonoids: hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharm. Drug Dispos. 29: 63-82 (2008). |
| Remarks | ※Time course of the metabolism of eriodictyol Tubes containing eriodictyol (1.5 mg) and an intestinal bacterial mixture (3 ml) were anaerobically incubated at intervals at 37°C. The mixture was adjusted to pH ca. 3 and extracted twice EtOAc (3 ml each). The products were loaded onto a Si gel tic plate, which was then developed with solvent system A. The metabolites were separated on the plate and quantitatively analyzed with a tic scanner at 240 nm to a reference wavelength of 550 nm by using calibration lines of authentic samples. The calibration lines were linear in a range of 1-40 μg/spot. [Hattori et al., J. Nat. Prod., 51, 874-878 (1988)] ※The majority of pharmacokinetic studies of individual flavonoids or after ingestion of foodstuffs have overlooked the chirality of some of these xenobiotics. In order to characterize for the first time the stereoselective pharmacokinetics of three flavonoids, hesperetin, naringenin and eriodictyol were intravenously administered (20 mg/kg) to male Sprague-Dawley rats, and their stereospecific content was assessed in various fruit juices. Concentrations in serum, urine and fruit juices were characterized via HPLC and verified by LC/MS. [Yanez et al., Biopharm. Drug Dispos., 29: 63-82 (2008)] ※Stereospecific pharmacokinetics of eriodictyol in serum after i.v. administration in rats (20 mg/kg) (mean±SEM, n=6) ※Stereospecific pharmacokinetics of hesperetin, and naringenin in serum after i.v. administration in rats (20 mg/kg) (mean±SEM, n=6) |

