|
|
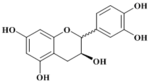
| Compound | Epicatechine |
| Animal species | human intestinal bacteria Eubacterium sp. strain SDG-2 |
| Metabolism parameters | |
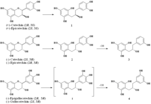
| Metabolites |
(+)-(2S 3S)-Epicatehin
(R)-2-(3-(3,4-dihydroxyphenyl)-2-hydroxypropyl)benzene-1,3,5-triol (-)-(2R, 3S)-Epicatechin (S)-2-(3-(3,4-dihydroxyphenyl)-2-hydroxypropyl)benzene-1,3,5-triol (S)-2-(2-hydroxy-3-(3-hydroxyphenyl)propyl)benzene-1,3,5-triol (-)-Epigallocatechin (2R, 3R) (S)-5-(2-hydroxy-3-(2,4,6-trihydroxyphenyl)propyl)benzene-1,2,3-triol (S)-2-(3-(3,5-dihydroxyphenyl)-2-hydroxypropyl)benzene-1,3,5-triol |
| Crude drug | Gambir |
| References | 1) Wang L., Meselhy M. R., Li Y., Nakamura N., Min B., Qin G. and Hattori M.: The heterocyclic ring fission and dehydroxylation of catechins and related compounds by Eubacterium sp. strain SDG-2, a human intestinal bacterium. Chem. Pharm. Bull., 49, 1640-1643 (2001). |
| Remarks | ・Incubation of (+)-epicatechin with Eubacterium sp. strain SDG-2 (+)-Epicatechin (1a) (10 mg in 1 ml MeOH) was anaerobically incubated with a bacterial suspension (20 ml) for 36 h. The reaction mixture was then treated as usual to give 2a (4 mg). [Wang et al., Chem. Pharm. Bull., 49, 1640-1643 (2001)] ・Incubation of (-)-epicatechin with E. sp. strain SDG-2 (-)-Epicatechin (1b) (30 mg in 1 ml MeOH) was anaerobically incubated with a bacterial suspension for 36 h. The reaction mixture was then treated as usual to give 2b (10 mg) and 3b (8 mg), respectively. [Wang et al., Chem. Pharm. Bull., 49, 1640-1643 (2001)] |

