|
|
| Compound | Paeoniflorin |
| Animal species | human intestinal bacteria, human intestinal microflora, Lactobacillus brevis, Bacteroides fragilis ss. Thetaotus |
| Metabolism parameters |
Oral administration
paeoniflorin
Oral administration paeoniflorin Oral administration Oral administration |
| Metabolites |
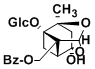
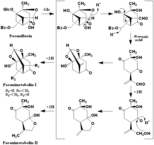
Paeoniflorin
((2S,2aR,2a1S,3aR,4R,5aR)-2a,4-dihydroxy-2-methylhexahydro-2H-1,5-dioxa-2,4-methanocyclobuta[cd]pentalen-2a1-yl)methyl benzoate ((1R,2S,5R,6S)-6-formyl-1,2-dihydroxy-2-methyl-4-oxobicyclo[3.1.1]heptan-6-yl)methyl benzoate 2-((1R,4S)-4-hydroxy-4-methyl-2,5-dioxocyclohexyl)acrylaldehyde (2S,3aR,5R,7aS)-3a-hydroxy-7a-methyl-8-methylenetetrahydro-2,5-methanobenzo[d][1,3]dioxol-6(3aH)-one Paeonimetabolin I (2S,5R)-2-hydroxy-5-(3-hydroxyprop-1-en-2-yl)-2-methylcyclohexane-1,4-dione (3aR,6S)-6,7a-dihydroxy-6-methyl-3-methylenehexahydrobenzofuran-5(6H)-one Paeonimetabolin II |
| Crude drug | Moutan Bark , Peony Root |
| References | 1) Hattori M., Shu Y. Z., Shimizu M., Hayashi T., Morita N., Kobashi K., Xu G. J. and Namba T.: Metabolism of paeoniflorin and related compounds by human intestinal bacteria. Chem. Pharm. Bull., 33, 3838-3846 (1985). 2) Shu Y. Z., Hattori M., Akao T., Kobashi K., Kakei K., Fukuyama K., Tsukihara T. and Namba T.: Metabolism of paeoniflorin and related compounds by human intestinal bacteria. II. Structures of 7S- and 7R-paeonimetabolins I and II formed by Bacteroides fragilis and Lactobacillus brevis. Chem. Pharm. Bull., 35, 3726-3733 (1987). 3) Shu Y. Z., Hattori M., Akao T., Kobashi K. and Namba T.: Metabolism of paeoniflorin and related compounds by human intestinal bacteria III. Metabolic ability of intestinal bacterial strains and fecal flora from different individuals. J. Med. Pharm. Soc. Wakan-Yaku, 4, 82-87 (1987). 4) Akao T., Shu Y. Z., Matsuda Y., Hattori M., Namba T. and Kobashi K.: Metabolism of paeoniflorin and related compounds by human intestinal bacteria. IV. Formation and structures of adducts of a metabolic intermediate with sulfhydryl compounds by Lactobacillus brevis. Chem. Pharm. Bull., 36, 3043-3048 (1988). 5) Takeda S., Wakui Y., Mizuhara Y., Ishihara K., Amagaya S., Maruno M. and Hattori M.: Gastrointestinal absorption of paeoniflorin in germ-free rats. J. Trad. Med., 13, 248-251 (1996). 6) Takeda S., Isono T., Wakui Y., Mizuhara Y., Amagaya S., Maruno M. and Hattori M.: In-vivo assessment of extrahepatic metabolism of paeoniflorin in rats: relevance to intestinal floral metabolism. J. Pharm. Pharmacol., 49, 35-39 (1997). 7) Heikal O. A., Miyashiro H., Akao T. and Hattori M.: Quantitative determination of paeoniflorin and its major metabolites, paeonimetabolin I, in the rat plasma by enzyme immunoassay. J. Trad. Med., 14, 15-19 (1997). 8) Heikal O. A., Akao T., Takeda S. and Hattori M.: Pharmacokinetic studies of paeonimetabolin I, a major metabolite of paeoniflorin from paeony roots. Biol. Pharm. Bull., 20, 517-521 (1997). 9) Heikal O. A., Kanaoka M., Akao T. and Hattori M.: Effects of spacer homologous and heterologous combinations on enzyme immunoassay for paeonimetabolin I, a major metabolite of paeoni florin. J. Trad. Med., 14, 105-113 (1997). 10) Meselhy M. R., Heikal O. A., Akao T., Hattori M., Ono H. and Sadakane C.: Disposition of paeoniflorin and paeonimetabolin I in rats after oral administration of Toki-Shakuyaku-San (TS) and Shakuyaku-Kanzo-To (SK). Natural Med., 52, 265-268 (1998). |
| Remarks |

