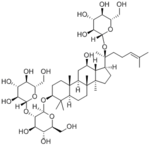
|
| Compound | Ginsenoside Rd |
| Animal species | human |
| Metabolism parameters | |
| Metabolites |
20(S)-Ginsenoside Rd
|
| Crude drug | Ginseng |
| References | 1) Xing Zeng, Yuanhui Deng, Yi Feng, Yiming Liu, Liu Yang, Yu Huang, Jing Sun, Weixiong Liang, and Yongyuan Guan, Pharmacokinetics and safety of ginsenoside Rd following a single or multiple intravenous dose in healthy chinese volunteers. J Clin Pharmacol, 50: 285-292 (2010). 2) Houfu Liu, Junling Yang, Feifei Du, Xiumei Gao, Xutao Ma, Yuhong Huang, Fang Xu, Wei Niu, Fengqing Wang, Yu Mao, Yan Sun, Tong Lu, Changxiao Liu, Boli Zhang, and Chuan Li, Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metabolism and Disposition, 37: 2290–2298 (2009). 3) Liu Yang, Yuanhui Deng, Shunjun Xu, Xing Zeng, In vivo pharmacokinetic and metabolism studies of ginsenoside Rd. Journal of Chromatography B, 854: 77–84 (2007). 4) Xiaoyu Li, Jianguo Sun, Guangji Wang, Haiping Hao, Yan Liang, Yuanting Zheng, Bei Yan and Longsheng Sheng, Simultaneous determination of panax notoginsenoside R1, ginsenoside Rg1, Rd, Re and Rb1 in rat plasma by HPLC/ESI/MS: platform for the pharmacokinetic evaluation of total panax notoginsenoside, a typical kind of multiple constituent traditional Chinese medicine. Biomedical Chromatography, 21: 735–746 (2007). DOI: 10.1002/bmc.813 5) Wei Wang, Guang-Ji Wang, Hai-Tang Xie, Jian-Guo Sun, Shuai Zhao, Xi-ling Jiang, Hao Li, Hua Lv, Mei-Juan Xu, Rui Wang, Determination of ginsenoside Rd in dog plasma by liquid chromatography–mass spectrometry after solid-phase extraction and its application in dog pharmacokinetics studies. Journal of Chromatography B, 852: 8–14 (2007). |
| Remarks | The pharmacokinetics and safety of ginsenoside Rd (Rd) were assessed in healthy Chinese volunteers. In the single dose study, a randomized, open-label, 3-way crossover design was used. Participants were assigned to receive 10, 45, or 75 mg Rd by intravenous infusion, with a 2-week washout period between dosing periods. Plasma levels of Rd were found to be proportional to dose, with the mean Cmax and AUC0–∞ ranging from 2.8 to 19.3 mg/L and 27.9 to 212.5 mg⋅h/L over the dose range studied. Ginsenoside Rd was slowly cleared from plasma (t1/2Z = 17.7-19.3 h). In the multiple-dose study, 10 mg Rd was administered once daily for 6 days. Slight drug accumulation was noted. The mean steady-state Cmax, AUC0–∞, and AUCss were 4.0 mg/L, 51.7 mg⋅h/L, and 26.4 mg⋅h/L, respectively. The t1/2Z was 20.5 h, which was similar to the single-dose value. [Zen et al., J Clin Pharmacol, 50: 285-292 (2010)] |

