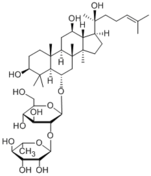
|
| Compound | Ginsenoside Rg2 |
| Animal species | rat |
| Metabolism parameters |
Intravenous administration Intravenous administration |
| Metabolites |
20(S)-Ginsenoside Rg2
20(R)-Ginsenoside Rg2 |
| Crude drug | Ginseng |
| References | 1) Fang-Jin Gui, Xiu-Wei Yang, Long-Yun Li, Jian-Ming Tian, Simultaneous enantiomer determination of 20 (R)- and 20 (S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method. Journal of Chromatography B, 850: 1–6 (2007). |
| Remarks | To analyze racemic 20 (R, S)-ginsenoside-Rg2, an anti-shock agent, a simple and reproducible high-performance liquid chromatographic (HPLC) method has been developed. The enantiomeric separation and determination were successfully achieved using a DiamonsilTM ODS C18 reversed-phase column with an RP18 guard column and a mobile phase of MeOH-aq. 4% H3PO4 (65:35, v/v, pH 5.1) with UV detection at 203 nm. Both enantiomers, 20 (R)-ginsenoside-Rg2 and 20 (S)-ginsenoside-Rg2, were well separated at 14.5 min and 13.6 min, respectively. In pharmacokinetic studies in rat plasma after intravenous administration of 20 (R, S)-ginsenoside-Rg2, the enantiomers were rapidly absorbed and eliminated. [Gui et al., Journal of Chromatography B, 850: 1–6 (2007)] Pharmacokinetic parameter of 20 (R)- and 20 (S)-ginsenoside-Rg2 [20 (R)- ginsenoside-Rg2 for 2 mg/kg and 20 (S)-ginsenoside-Rg2 for 23 mg/kg] in rat plasma (n=3) |

