|

|
| Compound | Homoorientin |
| Animal species | human intestinal microflora |
| Metabolism parameters | |
| Metabolites |
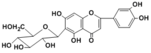
Homoorientin
Luteolin 6-C-Glucosyleriodictyol (±)-eriodictyol 3,4-dihydroxyphenylpropionic acid phloroglucinol Mangiferin Norathyriol Bergenin 4-O-Methylgallic acid |
| Crude drug | Swertia Herb |
| References | 1) Hattori M., Shu Y. Z., El-Sedawy A. I., Namba T., Kobashi K. and Tomimori T.: Metabolism of homoorientin by intestinal bacteria. J. Nat. Prod., 51, 874-878 (1988). |
| Remarks | ※Incubation of homoorientin (1) with an intestinal bacterial mixture Homoorientin (1, 400 mg) dissolved in DMSO (3 ml) was added to an intestinal bacterial mixture from human feces (420 ml). The mixture was incubated for 36 h at 37°C in an anaerobic jar, in which the air had been replaced with oxygen-free CO2 in the presence of activated steel wool (steel wool method). The mixture was adjusted to pH ca. 3 with 3 N HC1 in an ice bath and extracted three times with EtOAc (700 ml each). The EtOAc phase was washed with H2O, then concentrated in vacuo to give a residue (ca. 1 g). The residue was applied to a column of Si gel (90 g, 2.6 cm i.d. x 45 cm). The column was washed with C6H6 and eluted with C6H6-Et2O (5:1 and 3:1) to afford fractions of metabolite 3 (ca. 50 mg) and metabolite 5 (5 mg), respectively. Elution with CHCl3-MeOH (100:1 to 10:1) gave a fraction of metabolite 6 (13 mg). The crude metabolites 3 and 6 were further purified by repeated crystallization from C6H6/MeOH and MeOH/CHCl3, respectively. The crude metabolite 5 was purified by preparative tic. [Hattori et al., J. Nat. Prod., 51, 874-878 (1988)] ※Time course of the metabolism of homoorientin (1) Tubes containing homoorientin (1) (5 mg) and an intestinal bacterial mixture (5 ml) were incubated at intervals at 37°C in an anaerobic jar. The mixture was adjusted to pH ca. 3 and extracted successively with EtOAc and tert-BuOH (5 ml each). The products were then roughly separated by preparative tic with solvent system A, and the bands corresponding to the metabolites were extracted with MeOH (2 ml, twice). After filtration through a Millex-SR filter unit (0.5 μm, Millipore Corp.), the metabolites were quantitatively analyzed by hplc under the following conditions: mobile phase, MeOH-H2O-HOAc-5%H3PO4 (33:67:2:0.2) for compounds 1 and 2, and MeOH-H2O-HOAc -5% H3PO4 (48:52:2:0.2) for compounds 3-6; flow rate, 1.0 ml/min; detection, 280 nm. The calibration lines of the metabolites were prepared with authentic samples. [Hattori et al., J. Nat. Prod., 51, 874-878 (1988)] ※Time course of the metabolism of eriodictyol (3) Tubes containing eriodictyol (3) (1.5 mg) and an intestinal bacterial mixture (3 ml) were anaerobically incubated at intervals at 37°C. The mixture was adjusted to pH ca. 3 and extracted twice with EtOAc (3 ml each). The products were loaded onto a Si gel tic plate, which was then developed with solvent system A. The metabolites were separated on the plate and quantitatively analyzed with a tic scanner at 240 nm to a reference wavelength of 550 nm by using calibration lines of authentic samples. The calibration lines were linear in a range of 1-40 μg/spot. [Hattori et al., J. Nat. Prod., 51, 874-878 (1988)] |

