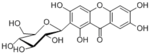
|

|
| Compound | Mangiferin |
| Animal species | human intestinal microflora, human intestinal bacteria Bacteroudes species MANG |
| Metabolism parameters | |
| Metabolites |
Mangiferin
Norathyriol |
| Crude drug | Anemarrhena Rhizome , Swertia Herb |
| References | 1) Hattori M., Shu Y. Z., Tomimori T., Kobashi K. and Namba T.: A bacterial cleavage of the C-glucosyl bond of mangiferin and bergenin. Phytochemistry, 28, 1289-1290 (1989). 2) Sanugul K., Akao T., Li Y., Kakiuchi N., Nakamura N. and Hattori M.: Isolation of a human intestinal bacterium that transforms mangiferin to norathyriol and inducibility of the enzyme that cleaves a C-glucosyl bond. Biol. Pharm. Bull., 28, 1672-1678 (2005). 3) Sanugul K., Akao T., Nakamura N., and Hattori M.: Two proteins, Mn2+, and low molecular cofactor are required for C-glucosyl-cleavage of mangiferin. Biol. Pharm. Bull., 28, 2035-2039 (2005). |
| Remarks | ※Metabolism of mangiferin (1) by human intestinal flora Mangiferin (1) was anaerobically incubated with a bacterial mixture from human feces, and a metabolite was isolated as a yellow powder, mp 300°C; UV λmax at 236, 254, 312 and 360 nm. High resolution MS: the molecular formula C13H18O6. IR, 1H NMR, 13C NMR and MS spectra were identical with those of authentic 1,3,6,7-tetrahydroxyxanthone (northyriol, 2). [Hattori et al., Phytochemistry, 28, 1289-1290 (1989)] ※Metabolism of mangiferin (1) by a defined bacterium from human feces A novel bacterium Bacteroudes species MANG was cultured with 0.5 mM mangiferin (1) in GAM broth under anaerobic conditions for 24 h. The metabolic product was extracted from 50 ml of the cultured broth with 50 ml of butanol 3 times. The extract was dried under vacuum, dissolved in 50% methanol, and then applied to preparative HPLC to purify the metabolite under the following conditions: Cosmosil 5C18-AR-II (20 × 250 mm) column (Nacalai Tesque, Kyoto, Japan); flow rate, 4 ml/min; detection, 260 nm; solvent system, 20―80% linear gradient of acetonitrile in 0.1% trifluoroacetic acid. [Sanugul et al., Biol. Pharm. Bull., 28, 1672-1678 (2005)] ※Qualitative assays of mangiferin (1) and norathyriol (2) by TLC and HPLC The butanol extract was separated by TLC (Silica gel RP-18 F254 S, Merck, Darmstadt, Germany) using CH3OH-H2O-CH3COOH (5:5:0.2) as a mixed solvent, and then mangiferin (1) and norathyriol (2) were visualized under UV light (Spectroline CM-10, Spectronics Corporation, NY, USA). The reversed phase HPLC (Shimadzu Co., Japan) conditions were as follows: recorder, C-R6A Chromatopac; pump, LC-6A; system controller, SCL-6B; monitor, SPD-6A; injector, SIL-9A; column, YMC-Pack ODS-AP AP-302 (4.6×150 mm) (YMC Co., Kyoto, Japan); flow rate, 1 ml/min; detection, 260 nm; solvent system, 10―40% acetonitrile linear gradient in 0.1% trifluoroacetic acid. The retention time of mangiferin (1) and norathyriol (2) were 7.5 and 14.5 min, respectively. [Sanugul et al., Biol. Pharm. Bull., 28, 1672-1678 (2005); Sanugul et al., Biol. Pharm. Bull., 28, 2035-2039 (2005)] ※Bacterial suspension (100 μl) precultured in GAM broth was cultivated in 5 ml of PYF broth containing 0.4 mM of mangiferin (1) at 37°C under anaerobic conditions. Two 100 μl portions were removed every 6 h. Bacterial growth was measured in one portion at 540 nm (absorbance) and mangiferin (1) and norathyriol (2) were quantified by HPLC. The acidified butanol extract (5 μl) of a 100 μl portion was dried in a Speed Vac SC 110 (Savant Instruments, NY, USA) and then dissolved in 100 μl of 50% methanol for HPLC application. [Sanugul et al., Biol. Pharm. Bull., 28, 1672-1678 (2005)] |

