|
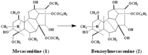
|
| Compound | Benzoylmesaconine |
| Animal species | rat |
| Metabolism parameters |
Intravenous administration Intravenous administration Oral administration Oral administration |
| Metabolites |
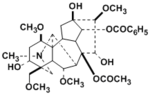
Mesaconitine
Benzoylmesaconine |
| Crude drug | Processed Aconite Root |
| References | 1) Zuo F., Zhao J., Nakamura N., Gao J. J., Akao T., Hattori M., Oomiga Y., and Kikuchi Y.: Pharmakokinetic stydy of benzoylmesaconine in rats using an enzyme immunoassay system. J. Nat. Med., 60, 313-321 (2006). 2) Zhao Z., Sun X. F., Nakamura N., Zuo F., Yang X. W., and Hattori M.: Development of an enzyme immunoassay system for mesaconitine and its application to the disposition study on mesaconitine. J. Trad. Med., 20, 201-207 (2003). |
| Remarks | ※Enzyme immunoassay for benzoylmesaconine (2) The immunization protocol included primary inoculation of three female albino rabbits with DBAE-BSA (2 mg) conjugate in saline (2 ml) emulsified with the same volume of complete Freund's adjuvant. After two weeks, the rabbits were continued with booster injections at two weeks for 3 months and three weeks' interval for two months. The emulsion was prepared with 1.5 mg of the conjugate in saline (1.5 ml) and incomplete Freund's adjuvant (1.5 ml). Samples or standard solutions (50 μl) containing various amounts of BM were incubated with an antiserum (50 μl) and a 103-fold diluted β-Gal conjugate (25 μl) at room temperature for 2 h. Ten-fold diluted goat anti-rabbit IgG (50 μl) and 100-fold diluted normal rabbit serum (20 μl) were then added to the reaction mixture. The mixture was left overnight at 4°C. After addition of buffer A (1 ml), the resulting mixture was centrifuged at 1100 × g for 20 min at 4°C. The supernatant was discarded, and the precipitates were washed with buffer A followed by incubation with 0.1 mM 4-methylumbelliferyl β-D-galactoside for 30 min at 30°C. The reaction was stopped by the addition of 100 mM glycine-NaOH buffer, pH 10.3 (3 ml). The fluorescence intensity of a product (4-methylumbelliferone) was spectrofluorometrically measured at wavelengths of 365 nm (excitation) and 448 nm (emission). [Zuo et al., J. Nat. Med., 60, 313-321 (2006)] ※Disposition of mesaconitine (1) after oral administration in rats One hour after oral administration of 1 at doses of 0.8, 0.2, 0.05 mg/kg in rats, under general anesthesia, the blood and spinal cord (L1-L4) were collected, and their mesaconitine (1) and benzoylmesaconine (2) contents were determined by the respective enzyme immunoassay systems. [Zuo et al., J. Nat. Med., 60, 313-321 (2006)] |

