|

|
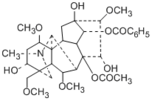
| Compound | Mesaconitine |
| Animal species | Wistar rat, human |
| Metabolism parameters |
Intravenous administration Oral administration |
| Metabolites |
Mesaconitine
Benzoylmesaconine Mesaconine |
| Crude drug | Aconite Root , Processed Aconite Root |
| References | 1) Zhao Z., Sun X. F., Nakamura N., Zuo F., Yang X. W., and Hattori M.: Development of an enzyme immunoassay system for mesaconitine and its application to the disposition study on mesaconitine. J. Trad. Med., 20, 201-207 (2003). 2) Zuo F., Zhao J., Nakamura N., Gao J. J., Akao T., Hattori M., Oomiga Y., and Kikuchi Y.: Pharmakokinetic stydy of benzoylmesaconine in rats using an enzyme immunoassay system. J. Nat. Med., 60, 313-321 (2006). 3) Fan Zhang, Ming-hai Tang, Li-juan Chen, Rui Li, Xian-huoWang, Jun-guo Duan, Xia Zhao, Yu-quanWei: Simultaneous quantitation of aconitine, mesaconitine, hypaconitine, benzoylaconine, benzoylmesaconine and benzoylhypaconine in human plasma by liquid chromatography–tandem mass spectrometry and pharmacokinetics evaluation of “SHEN-FU” injectable powder. J. Chromato. B, 873, 173–179 (2008). |
| Remarks | ※Standard curve of mesaconitine (1) 2.5×104-fold diluted As-DEAG in 50 μl of buffer A, 50 μl of serially diluted mesaconitine (5×10-4-50 ng/tube) or 50 μl of buffer A as a blank, and 25 μl of 103-fold diluted DEAG-β-Gal were incubated at room temperature for 2 h. Afterward, 20 μl of 102-fold diluted normal rabbit serum and 50 μl of 10-fold diluted goat antiserum to rabbit Ig G were added to the reaction system, which was allowed to stand at 4 °C overnight. After measurement of fluorescence, B/B0 values were calculated as the percentage of the enzyme activity of the labeled antigen bound to the antiserum in the presence of various concentrations of mesaconitine (B) to that in the absence of mesaconitine (B0). [Zhao et al., J. Trad. Med., 20, 201-207 (2003)] ※Measurement of plasma concentration of mesaconitine (1) after intravenous and oral administration Seven-week old male Wistar rats about 220 g each, were fasted one day before the pharmacokinetic study. After intravenous administration at a dose of 0.005 mg/kg or oral administration at a dose of 1 mg/kg to rats, blood was collected by haperinated capillaries at 5 min, 15 min, 0.5 h, 1 h, 2 h, 4 h and 8 h after injection or continued at 12 h and 24 h after oral administration. [Zhao et al., J. Trad. Med., 20, 201-207 (2003)] ※Disposition of mesaconitine (1) after oral administration in rats One hour after oral administration of mesaconitine (1) at doses of 0.8, 0.2, 0.05 mg/kg in rats, under general anesthesia, the blood and spinal cord (L1-L4) were collected, and the samples treated as described above. [Zuo et al., J. Nat. Med., 60, 313-321 (2006)] “SHEN-FU” injectable powder was applied to 18 healthy volunteers by intravenous drop infusion. Six volunteers were involved in each experiment. The blood samples were collected at intervals after intravenous drop infusion. The pharmacokinetics demonstrated that the concentrations of aconitine, mesaconitine, and hypaconitine were at very low levels with < 0.2, < 0.2, and < 0.7 ng/mL or under detection limit for all) and the content of benzoylmesaconine was highest. [Zhang et al., J. Chromato. B, 873, 173–179 (2008).] |

