|
|
| Compound | Hypaconitine |
| Animal species | human |
| Metabolism parameters | |
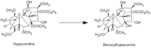
| Metabolites |
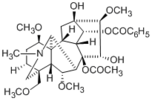
Hypaconitine
Benzoylhypaconine |
| Crude drug | Processed Aconite Root |
| References | 1) Fan Zhanga, Ming-hai Tanga, Li-juan Chena, Rui Li, Xian-huoWang, Jun-guo Duan, Xia Zhao, Yu-quanWei, Simultaneous quantitation of aconitine, mesaconitine, hypaconitine, benzoylaconine, benzoylmesaconine and benzoylhypaconine in human plasma by liquid chromatography–tandem mass spectrometry and pharmacokinetics evaluation of “SHEN-FU” injectable powder. J. Chromato. B, 873, 173–179 (2008). 2) Ling Ye, Tao Wang, Caihua Yang, Lan Tang, Juan Zhou, Chang Lv, Yun Gong, Zhihong Jiang, Zhongqiu Liu, Microsomal cytochrome P450-mediated metabolism of hypaconitine, an active and highly toxic constituent derived from Aconitum species. Toxicology Letters 204, 81–91 (2011). 3) Yang Yang, Juan Chen, Yan-Ping Shi, Determination of aconitine, hypaconitine and mesaconitine in urine using hollow fiber liquid-phase microextraction combined with high-performance liquid chromatography. Journal of Chromatography B, 878, 2811–2816 (2010). 4) Bo Sun, Shengming Wu, Ling Li, Haijing Li, Qi Zhang, Hebing Chen, Famei Li, Fangting Dong and Xianzhong Yan, A metabolomic analysis of the toxicity of Aconitum sp. alkaloids in rats using gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 23, 1221–1228 (2009). |
| Remarks | ・“SHEN-FU” injectable powder were applied to 18 healthy volunteersby intravenous drop infusion. Six volunteers were involved in each experiment. The blood samples were collected at intervals after intravenous drop infusion. The pharmacokinetics demonstrated that the concentrations of aconitine, mesaconitine, and hypaconitine were at very low levels with < 0.2, < 0.2, and < 0.7 ng/mL or under detection limit for all) and the content of benzoylmesaconine was highest. [Fan Zhang et al., J. Chromato. B, 873, 173–179 (2008).] |

