|

|
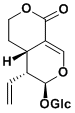
| Compound | Sweroside |
| Animal species | human intestinal microflora, human intestinal bacteria Proteus mirabilis |
| Metabolism parameters | |
| Metabolites |
Sweroside
(4aS,5R,6R)-6-hydroxy-5-vinyl-4,4a,5,6-tetrahydropyrano[3,4-c]pyran-1(3H)-one 2-((S,E)-3-(hydroxymethylene)-2-oxotetrahydro-2H-pyran-4-yl)but-3-enal (Z)-2-((S,E)-3-(hydroxymethylene)-2-oxotetrahydro-2H-pyran-4-yl)but-2-enal Naucledal Epinaucledal (4aS,5R,6S)-5-(hydroxymethyl)-6-methyl-4,4a,5,6-tetrahydropyrano[3,4-c]pyran-1(3H)-one |
| Crude drug | Cornus Fruit , Swertia Herb |
| References | 1) Adel I. El-Sedawy A. I., Hattori M., Kobashi K., and Namba T.: Metabolism of sweroside from Swertia japonica by human intestinal bacteria. Shoyakugaku Zasshi 44, 122-126 (1990). |
| Remarks | ※Screening of defined strains for ability to metabolize sweroside (1) Each pre-cultured bacterial suspension (0.2 ml) was added to GAM broth (10 ml) and cultured for 24 h at 37°C in an anaerobic jar, according to the steel wool method. Sweroside (8.4 mg, 1) was added to each culture, and the mixture was incubated for 24 h under anaerobic conditions and then extracted with EtOAc (10 ml). After evaporation of the EtOAc, CHCl3-MeOH (1:1, 0.5 ml) was added and an aliquot (30 μl) of the solution was applied to a silica gel TLC plate, which was developed with CHCl3-MeOH (17:1). The spots separated on the plate were quantitatively analyzed with a TLC-scanner at 245 nm. After the EtOAc extraction, the remaining aqueous solution was evaporated in vacuo to give a residue, to which MeOH (0.5 ml) was added. An aliquot (20 μl) of the MeOH solution was spotted on a TLC plate and the plate was developed with CHCl3-MeOH (5:1). The amount of sweroside (1) recovered was quantitatively analyzed by TLC-densitometry. [Adel et al., Shoyakugaku Zasshi 44, 122-126 (1990)] ※Metabolism of sweroside (1) by Proteus mirabilis A precultured bacterial suspension (100 ml) of P. mirabilis was added to GAM broth (900 ml) and cultured for 24 h at 37°C under anaerobic conditions. The bacterial culture was centrifuged at 7000 rpm for 10 min. The pellets were suspended in 0.1 m phosphate buffer (100 ml, pH 7.3). Sweroside (200 mg, 1) was added to the suspension (80 ml). The mixture was anaerobically incubated for 22 h at 37°C and extracted 3 times with EtOAc (100 ml each). The combined EtOAc phases were evaporated to dryness in vacuo. The residue was chromatographed on preparative TLC plates with CHCl3 (17:1), and the metabolite (metabolite A; 2) obtained was purified further by high-performance liquid chromatography (HPLC; Chemo Pak, nucleosil 50-5, 250 mm x 4.6 mm ID) with CHCl3-MeOH (17:1) as a mobile phase. Yield after purification, 3.5 mg. [Adel et al., Shoyakugaku Zasshi 44, 122-126 (1990)] |

