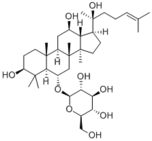
|
| Compound | Ginsenoside Rh1 |
| Animal species | mouse |
| Metabolism parameters |
Intravenous administration Intragastric administration |
| Metabolites |
20(S)-Ginsenoside Rh1
|
| Crude drug | Ginseng |
| References | 1) Li Lai, Haiping Hao, Yitong Liu, Chaonan Zheng, QiongWang, Guangji Wang, Xijin Chen, Characterization of pharmacokinetic profiles and metabolic pathways of 20(S)-ginsenoside Rh1 in vivo and in vitro. Planta Med., 75: 797–802 (2009). |
| Remarks | The absolute bioavailability of 20(S)-ginsenoside Rh1 in rats was only 1.01%. After intragastrical administration of ginsenoside Rh1, two mono-oxygenated metabolites were detected from the urine, bile, liver tissue, and intestinal tract content, while the de-glucosylated product, 20(S)-protopanaxatriol, was only found in the contents of the intestinal tract. An in vitro incubation study confirmed that the CYP450-catalyzed mono-oxygenation, the intestinal bacteria mediated de-glucosylation, and the gastric acid mediated hydration reaction were the main metabolic pathways of 20(S)-ginsenoside Rh1. [Lai et al., Planta Med., 75: 797–802 (2009)] The main pharmacokinetic parameters of Rh1 after i. v. (5 mg/kg) and i. g. (50mg/kg) administrations (mean ± S.D., n = 4). CL/F: apparent oral total body clearance (where F represents bioavailability); CL: total clearance; Cmax: the maximum plasma concentration; F: bioavailability; MRT: mean retention time; T1/2α: the absorption half-life time; T1/2β: the apparent elimination half-life time; Tmax: the time to reach Cmax; Vd: the apparent volume of distribution. [Lai et al., Planta Med., 75: 797–802 (2009)] |

