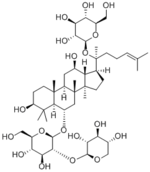
|
| Compound | Notoginsenoside R1 |
| Animal species | rat |
| Metabolism parameters |
Intravenous administration Intravenous administration Intravenous administration Intravenous administration Intravenous administration |
| Metabolites |
Notoginsenoside R1
|
| Crude drug | Acanthopanax Bark |
| References | 1) Xiaoyu Li, Jianguo Sun, Guangji Wang, Haiping Hao, Yan Liang, Yuanting Zheng, Bei Yan and Longsheng Sheng, Simultaneous determination of panax notoginsenoside R1, ginsenoside Rg1, Rd, Re and Rb1 in rat plasma by HPLC/ESI/MS: platform for the pharmacokinetic evaluation of total panax notoginsenoside, a typical kind of multiple constituent traditional Chinese medicine. Biomedical Chromatography, 21: 735–746 (2007). DOI: 10.1002/bmc.813 |
| Remarks | The HPLC/ESI/MS technique provided an excellent method for the simultaneous quantification of R1, Rg1, Rd, Re and Rb1 in rat plasma and was successfully applied to the pharmacokinetic study of a multiple-constituent traditional Chinese medicine, total panax notoginsenoside (Xuesaitong injection). [Li et al., Biomedical Chromatography, 21: 735–746 (2007)] The main pharmacokinetic parameters of panax notoginsenoside R1 and ginsenoside Rg1, Rd, Re and Rb1 after intravenous administration of 10 mg/kg dosage of Xuesaitong injection (TPNS) in rat plasma (n = 6) R1, notoginsenoside R1; Rg1, ginsenoside Rg1; Rd, ginsenoside Rd; Re, ginsenoside Re; Rb1, ginsenoside Rb1 [Li et al., Biomedical Chromatography, 21: 735–746 (2007)] |

