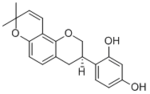
|
| Compound | Glabridin |
| Animal species | human |
| Metabolism parameters |
Oral administration Oral administration Oral administration |
| Metabolites |
Glabridin
|
| Crude drug | Glycyrrhiza |
| References | 1) Fumiki Aoki, Kaku Nakagawa, Mitsuaki Kitano, Hideyuki Ikematsu, Kenjirou Nakamura, Shinichi Yokota, Yuji Tominaga, Naoki Arai, and Tatsumasa Mae, Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. Journal of the American College of Nutrition, 26, 209–218 (2007). |
| Remarks | Licorice flavonoid oil (LFO: Kaneka Glavonoid Rich Oil™) is a new dietary ingredient containing licorice flavonoids dissolved in medium-chain triglycerides (MCT). A single-dose and two multiple-dose studies at low (300 mg), moderate (600 mg) and high (1200mg) daily doses of LFO were carried out using a placebo-controlled single-blind design. Pharmacokinetic analysis in the single-dose study with healthy male subjects (n= 5) showed that glabridin was absorbed and reached the maximum concentration (Cmax) after approximately 4 h (Tmax), and then eliminated relatively slowly in a single phase with a T1/2 of approximately 10 h at all doses. The Cmax and AUC0–24 h increased almost linearly with dose. The multiple-dose studies with healthy male and female subjects for 1 week and 4 weeks suggested that plasma glabridin reached steady state levels within 2 weeks with a single daily administration of 300 to 1200 mg/day LFO. [Aoki et al., Journal of the American College of Nutrition, 26, 209–218 (2007)] Pharmacokinetic parameters of glabridin in healthy male subjects after a single oral administration of licorice flavonoid oil (LFO)a,b |

